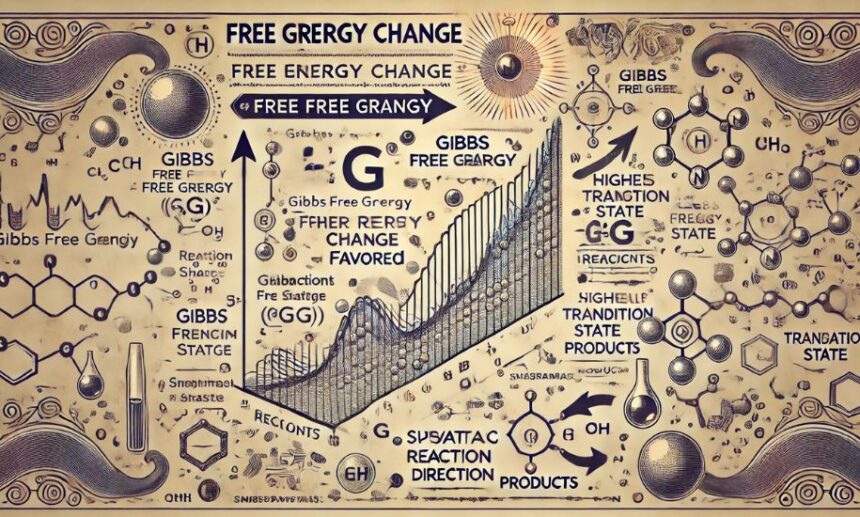
why is higher change in free energy favored in thermodynamic Thermodynamics is a fundamental aspect of physics and chemistry, providing deep insights into how energy governs all processes in the universe. One of the key concepts in thermodynamics is free energy change, particularly Gibbs free energy (ΔG). In many natural and industrial processes, a higher change in free energy is favored due to its critical role in determining the spontaneity, efficiency, and direction of reactions.
In this article, Physics Heaven explores why higher change in free energy is favored in thermodynamic, detailing the principles behind energy transformations, reaction spontaneity, equilibrium, and the efficiency of chemical and physical processes.
Understanding Free Energy and Thermodynamics
Thermodynamics is the study of energy transformations and their effect on matter. Free energy, specifically Gibbs free energy (G), is a thermodynamic quantity that predicts whether a reaction will occur spontaneously.
The change in Gibbs free energy (ΔG) is given by the equation:
ΔG=ΔH−TΔSΔG = ΔH – TΔS
where:
- ΔH = Enthalpy change (heat exchange in the reaction)
- T = Temperature in Kelvin
- ΔS = Entropy change (disorder in the system)
If ΔG is negative, the process is spontaneous, meaning it will proceed without requiring external energy input. A higher change in free energy in thermodynamic means a stronger driving force for reactions, making the process more efficient and productive.
Why is Higher Change in Free Energy Favored in Thermodynamic?
A greater change in free energy in thermodynamics is favored for several key reasons, all of which influence the behavior of physical, chemical, and biological systems. Let’s explore these factors in detail.
1. Spontaneity of Reactions and Favorable Energy Conditions
One of the most important reasons why higher change in free energy is favored in thermodynamic is because it ensures spontaneity. When a reaction has a large negative ΔG, it means there is a strong energy release, making the reaction more likely to occur naturally.
For example, in combustion reactions, a large negative ΔG ensures a spontaneous and highly exothermic reaction, releasing heat and energy that can be used for power generation, industrial processes, or even biological metabolism.
2. Stability and Favorability of Products Over Reactants
Another critical reason why higher change in free energy is favored in thermodynamic is that it drives systems towards a more stable state. Reactions proceed in a way that lowers the system’s overall free energy, meaning that products formed in a reaction are usually more stable than the reactants.
For instance, in biological systems, ATP (adenosine triphosphate) hydrolysis has a highly negative ΔG, which makes it a powerful energy source for cellular metabolism, muscle contraction, and biochemical reactions.
3. Equilibrium Shift and Reaction Completion
In thermodynamic systems, the position of equilibrium is heavily influenced by free energy. A higher change in free energy helps push the reaction farther toward product formation, shifting the equilibrium in favor of more complete reactions.
Consider industrial processes like the Haber process for ammonia production. By maximizing free energy change, conditions are optimized to ensure the maximum yield of ammonia, making the process economically and practically viable.
4. Energy Efficiency in Chemical and Physical Processes
A higher change in free energy allows for more efficient energy utilization. This is crucial in thermodynamic applications like power plants, batteries, and biological energy cycles.
For example, in fuel cells, a large ΔG change means that the reaction is more efficient at converting chemical energy into electrical energy, leading to better battery performance and lower energy waste.
Similarly, in the physics of phase transitions, substances tend to change from higher-energy states to lower-energy states, following thermodynamic laws that favor greater free energy changes.
5. Driving Non-Spontaneous Reactions with Coupled Reactions
Another reason why higher change in free energy is favored in thermodynamic is that it allows for coupling reactions. Many biological and chemical processes require input energy, but they can be driven by high-energy, spontaneous reactions.
For instance, in cellular respiration, the breakdown of glucose has a large negative ΔG, which helps drive ATP synthesis, even though ATP formation itself is not spontaneous. By coupling reactions with a greater free energy change, biological systems ensure efficient energy transfer.
6. Impact on Industrial and Technological Applications
The concept of free energy change is not just theoretical—it plays a vital role in engineering, chemistry, and physics-based industries. Whether it’s in the design of thermodynamic engines, optimizing reaction conditions, or improving energy efficiency in industrial plants, a higher free energy change ensures better performance and sustainability.
In processes like metallurgy, electrochemistry, and environmental engineering, maximizing free energy change helps increase yield, reduce waste, and improve efficiency, making industries more cost-effective and sustainable.
7. The Role of Temperature and Entropy in Free Energy Change
Temperature and entropy significantly influence free energy change. At higher temperatures, the entropy term TΔS becomes more dominant, making processes with high entropy increases more thermodynamically favored.
For instance, in gas expansion and evaporation, a higher entropy change (ΔS) contributes to a large free energy change, ensuring that the reaction occurs more favorably at higher temperatures.
This is one reason why boiling, evaporation, and dissolution processes are strongly dependent on temperature variations.
Final Thoughts: Why is Higher Change in Free Energy Favored in Thermodynamic?
The principles of thermodynamics revolve around energy transformations, and higher changes in free energy play a fundamental role in determining the spontaneity, direction, and efficiency of processes.
From chemical reactions to biological metabolism and industrial applications, a greater free energy change ensures optimal performance, energy utilization, and reaction feasibility. This is why, in thermodynamic systems, maximizing free energy change is a key strategy for achieving efficiency, stability, and sustainability.
At Physics Heaven, we continue to explore these fascinating principles to help students, researchers, and enthusiasts understand the power of thermodynamics in shaping our universe.