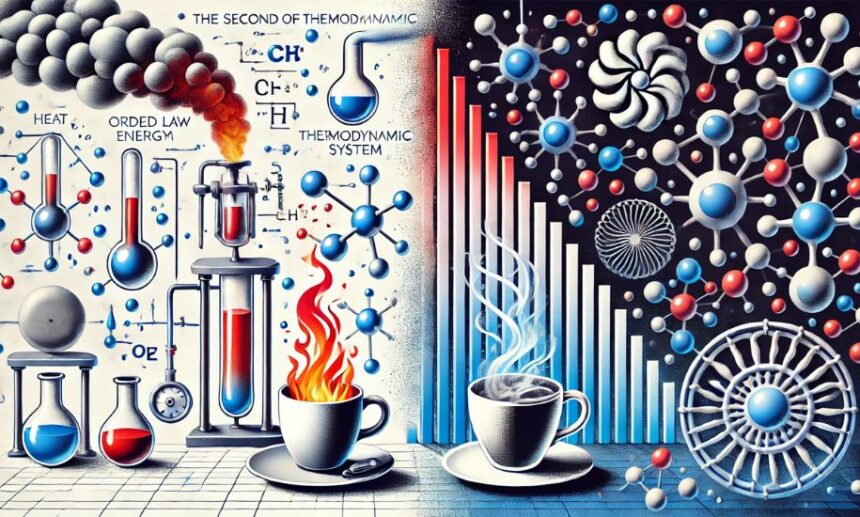
Physics Heaven welcomes you to an insightful discussion on one of the most fundamental principles in physics: the second law of thermodynamics states that energy tends to disperse and systems naturally progress towards disorder. This law plays a crucial role in our understanding of energy transfer, entropy, and the limitations of energy efficiency in various systems.
In this article, we will explore the detailed meaning of the second law of thermodynamics states that, its implications in different fields, and real-world examples demonstrating its significance.
What Does the Second Law of Thermodynamics State?
The second law of thermodynamics states that in any natural thermodynamic process, the total entropy of a closed system will either increase or remain constant but never decrease. In simpler terms, entropy, which measures the disorder of a system, always tends to rise. This means that energy transformations are never 100% efficient, and some energy is always lost as unusable heat.
This law has far-reaching consequences, governing everything from heat engines to biological processes and the universe’s ultimate fate. Understanding how the second law of thermodynamics states that energy disperses helps scientists and engineers design more efficient systems.
Entropy: The Measure of Disorder
Entropy is a key concept in thermodynamics, and it explains why the second law of thermodynamics states that systems evolve towards disorder. The term “entropy” refers to the amount of molecular randomness or chaos in a system.
For instance:
- A hot cup of coffee left in a room will eventually cool down, as heat spreads into the surroundings.
- A gas released in a container will spread evenly rather than staying in one corner.
- A well-organized room, if left untouched, will naturally become messy over time.
These examples illustrate how the second law of thermodynamics states that entropy increases naturally in an isolated system.
Heat Transfer and Energy Flow
Heat transfer is another crucial aspect of the second law of thermodynamics states that energy moves from a region of higher temperature to one of lower temperature until equilibrium is reached. There are three main methods of heat transfer:
- Conduction: Direct transfer of heat through materials, such as a metal rod heated at one end.
- Convection: Movement of fluids (liquids or gases) carrying heat, like warm air rising and cool air sinking.
- Radiation: Emission of energy as electromagnetic waves, such as sunlight warming the Earth.
Each of these processes demonstrates how the second law of thermodynamics states that heat energy spreads out over time.
The Second Law of Thermodynamics in Real-Life Applications
The principles of the second law of thermodynamics states that are evident in various practical applications, including:
1. Heat Engines and Power Plants
In engines, fuel is burned to produce heat, which is then converted into mechanical work. However, because the second law of thermodynamics states that energy cannot be fully converted without losses, some energy is always wasted as heat. This is why no engine is 100% efficient.
2. Refrigerators and Air Conditioners
These devices work against natural entropy increase by using external energy (electricity) to transfer heat from cooler regions to warmer ones. However, due to the inefficiencies dictated by the second law of thermodynamics states that, energy is still lost as waste heat.
3. Biological Systems
Living organisms consume energy to maintain order in their bodies. However, according to the second law of thermodynamics states that, this energy is ultimately lost as heat, contributing to the overall increase in entropy in the environment.
4. Cosmic Evolution
The universe itself follows the second law of thermodynamics states that principle. Over time, energy spreads out, and eventually, the universe may reach a state of “heat death,” where all usable energy is evenly distributed and no work can be performed.
The Arrow of Time and Irreversibility
One profound implication of the second law of thermodynamics states that is the concept of the “arrow of time.” Because entropy always increases, time moves in one direction—towards disorder. This explains why broken glass does not spontaneously reassemble and why we remember the past but not the future.
Challenges and Misconceptions
Despite its broad acceptance, there are some misunderstandings about the second law of thermodynamics states that:
- It does not violate energy conservation – The law does not say energy is lost, only that it becomes less usable.
- It applies to closed systems – In open systems, energy can enter and leave, which can temporarily decrease entropy locally (e.g., plants using sunlight for photosynthesis).
- Reversible processes are idealized concepts – In reality, all processes have inefficiencies and increase entropy.
Conclusion: The Universal Importance of the Second Law
Physics Heaven emphasizes that the second law of thermodynamics states that energy disperses, and entropy increases, shaping how systems evolve. From engineering applications to the cosmos, this fundamental principle governs the behavior of energy and matter.
Understanding the second law of thermodynamics states that helps scientists improve energy efficiency, develop sustainable technologies, and explore the universe’s fate. Whether in everyday occurrences or advanced physics, this law remains one of the most profound discoveries in science.