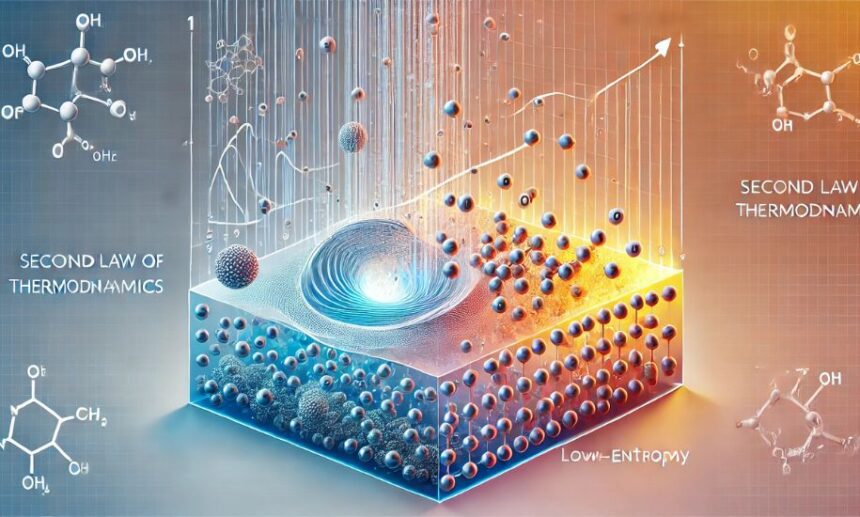
Physics Heaven brings you an insightful exploration of the “picture of second law of thermodynamics,” a fundamental principle that governs the natural world. This law plays a crucial role in understanding entropy, energy dispersion, and the irreversible nature of processes. By visualizing this concept, we can better comprehend its implications in physics, engineering, and daily life.
Understanding the Picture of Second Law of Thermodynamics
The second law of thermodynamics states that the total entropy of an isolated system always increases over time. Entropy, often referred to as the measure of disorder, determines the natural direction of energy transfer. The “picture of second law of thermodynamics” helps illustrate how energy spontaneously flows from ordered to disordered states. This visualization is crucial in physics, as it provides a clear representation of the irreversible processes in nature.
Visual Representation of Entropy and Energy Flow
One of the most effective ways to grasp the second law is through its pictorial representation. A typical image illustrating the “picture of second law of thermodynamics” shows:
- A system with two compartments, one hot and one cold.
- Heat flowing from the hot region to the cold region.
- Increased molecular disorder over time.
This visual representation captures the essence of entropy increase, a key aspect of the second law. Whether it’s melting ice, burning fuel, or even biological decay, the “picture of second law of thermodynamics” provides an intuitive understanding of why these processes occur.
How the Picture of Second Law of Thermodynamics Explains Irreversibility
The irreversibility of natural processes is a direct consequence of the second law. Imagine a broken cup or a burnt piece of wood—these cannot spontaneously return to their original state. The “picture of second law of thermodynamics” typically depicts this concept by showing a system evolving from order to disorder.
For example:
- A visual of gas molecules spreading in an empty room.
- A hot cup of coffee gradually cooling down.
- A burning candle releasing heat and gases.
These images reinforce the idea that energy transformations lead to increased entropy, making the process irreversible.
Applications of the Picture of Second Law of Thermodynamics in Daily Life
Physics Heaven emphasizes the importance of this concept in everyday experiences. The “picture of second law of thermodynamics” is not just an abstract idea but a principle that governs numerous real-world applications:
1. Thermodynamics in Heat Engines
A commonly used diagram for the “picture of second law of thermodynamics” shows heat engines, such as steam engines and car engines, where energy is transferred from a hot reservoir to a cold one. This representation helps engineers optimize energy efficiency and minimize entropy loss.
2. Climate and Weather Systems
Meteorological illustrations often use the “picture of second law of thermodynamics” to explain how heat moves through the atmosphere. For example, weather maps depicting ocean currents and wind patterns demonstrate energy transfer from high to low-temperature regions.
3. Biological Processes and Life Systems
The second law also applies to biological systems. Diagrams showcasing food digestion, respiration, and even the aging process represent the constant increase in entropy. The “picture of second law of thermodynamics” thus plays a vital role in understanding metabolic processes.
Entropy and the Universe: A Cosmic Picture of Second Law of Thermodynamics
On a grand scale, the universe itself follows the second law. The “picture of second law of thermodynamics” in cosmology often illustrates:
- The expanding universe gradually increasing in entropy.
- The transformation of stars as they burn their fuel.
- The concept of heat death, where the universe reaches a state of maximum entropy.
These cosmic representations help scientists and enthusiasts alike grasp the irreversible nature of time and energy distribution in the universe.
Why the Picture of Second Law of Thermodynamics Matters
Physics Heaven believes that visual learning enhances comprehension. The “picture of second law of thermodynamics” is a powerful tool that simplifies complex thermodynamic concepts. By using diagrams and graphical illustrations, students, engineers, and researchers can better analyze:
- Energy efficiency in machines.
- Environmental heat exchange processes.
- The progression of entropy in various systems.
Through the “picture of second law of thermodynamics,” we gain a deeper appreciation for the natural order and the irreversible processes that shape our world.
Conclusion: Embracing the Picture of Second Law of Thermodynamics
The second law of thermodynamics is an essential principle that explains energy transformation and entropy increase. Visualizing this law through diagrams and illustrations provides a clear understanding of how nature progresses from order to disorder. Physics Heaven encourages learners to embrace the “picture of second law of thermodynamics” as a key educational tool in mastering thermodynamics.